Thermochemistry: Energy and Energy Transfer
The study of energy changes that accompany physical and chemical changes
- Temperature vs Heat (q)
- Temperature is avg kinetic energy of particles. Relates to How Entities Move. ONLY relates to speed
- Heat: the overall thermal energy in a sample.
- Depends on:
- Temperature * number of particles (mass) * type of solid particles (s/l/g)
- Systems
- Anything that’s not in the system is a surrounding
- Open: both energy and matter can be exchanged with surroundings (my room)
- Closed: Only energy (sealed metal drum)
- Isolated: neither energy nor matter (e.g. ISS/Thermus)
- Exothermic vs Endothermic
- Exo: Gives off heat (hot pack)
- Edo: Absorbs heat (cold pack)
- When you touch something, you feel thermal conductivity of the substance not simply it’s temperature.
Specific Heat Capacity
the heat required to raise the temperature of the unit mass of a given substance by a given amount (usually one degree)
Measuring Energy changes
where:
- is the energy in J or
- is the mass in g or
- is the specific heat capacity in or
- is the change in temperature () in
Calorimeter
Isolated system. Food is burned to calculate calories
Bomb Calorimeter
(used to calculate higher energy items by blowing them up)
Energy Transfer
Energy is always transferred from an object of higher temperature to an object of a lower temperature until they are equal
Methods
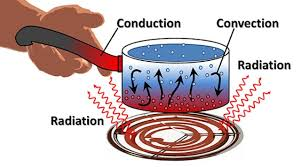
- Conduction - transfer through direct touch
- Convection - transfer through fluids
- Radiation - transfer through space (no direct contact)
Calculations
The First Law of Thermodynamics
The total energy in the universe is constant therefore, the change in energy of the universe is
Phases
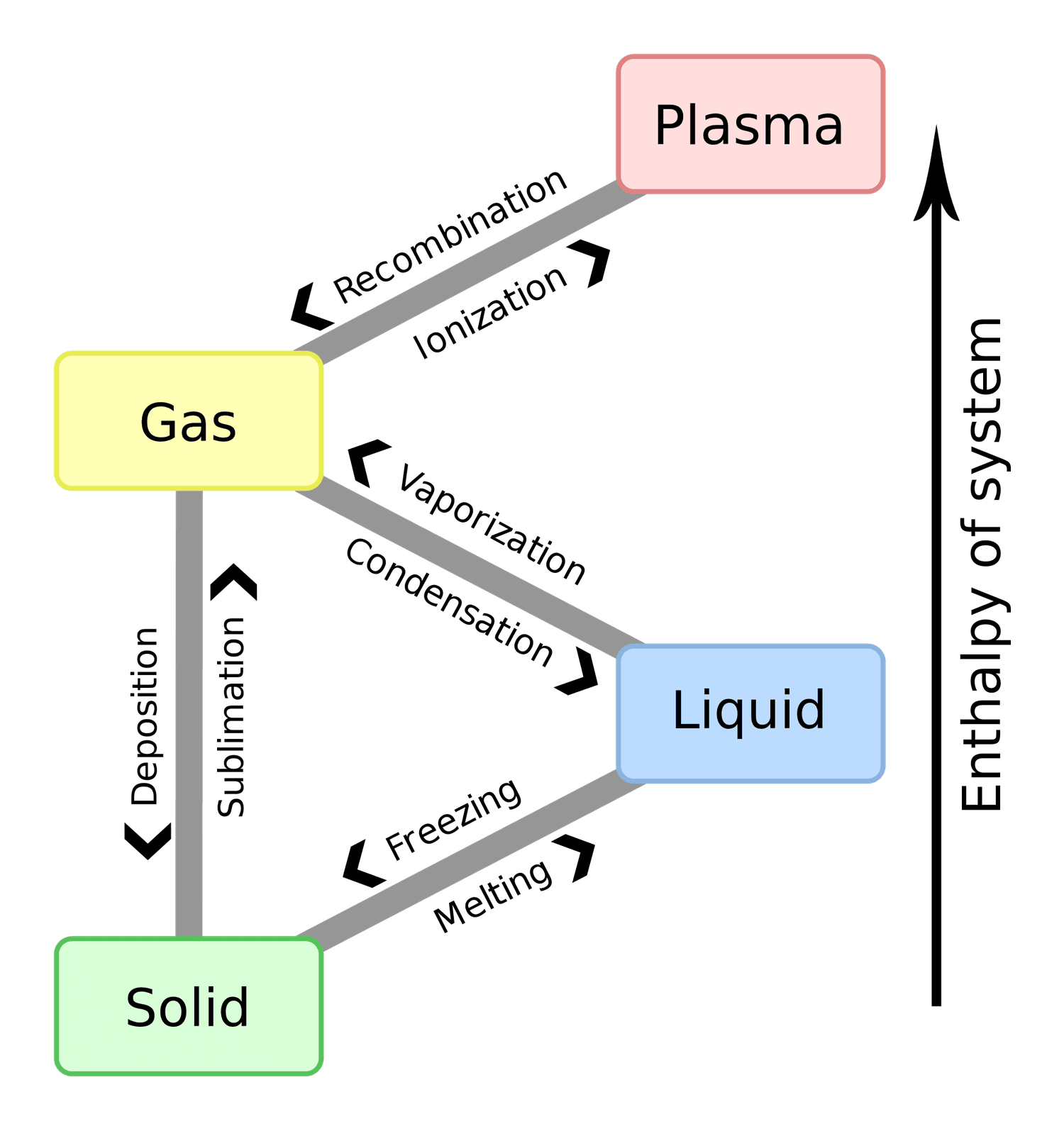
Calculating Energy Across Phase Changes
-
Break the process into segments: heating, phase changes, and cooling.
-
For temperature changes, use (specific heat), where is in joules. Convert to kJ by dividing by .
-
For phase changes, use , where is the lat ent heat (fusion or vaporization) in kJ/mol.
-
Combine all contributions:
-
Ensure consistent units (mass in grams, in J/g°C, in °C, in kJ/mol).
Latent heat
Enthalpy
H, is the heat content of the system
You can’t calculate the total enthalpy of a system but we can measure the change,
if is zero, then
Enthalpy is the amount of heat produced during a change in state or chemical reaction. It’s represented in and generally has the unit of
is also equal to the difference in enthalpies in products and reactants
Types of enthalpies
Represented by subscript
2. Enthalpy of fusion - melting/freezing phase change
3. Enthalpy of vaporisation - evaporation/condensation phase change
4. Enthalpy of reaction - energy change involved in a reaction
5. Enthalpy of combustion - energy released when of fuel undergoes combustion
6. Enthalpy of formation - energy required to produce of a chemical from it’s elements
Thermochemical equation
A chemical reaction + energy
e.g.
If the is negative (exothermic), then the energy is on the products side, if positive (endothermic) it would be on the reactants side.
Standard Molar Enthalpy,
The same reaction carried out in different physical states can have Different
i.e. change in state has an effect on the enthalpy. This is due to some energy required to do phase changes
even different temps/pressures can effect the enthalpy of action,
This is due to the fact that temp/pressure effect the state of a substance.
- We usually use for this reason
- And when we use , we represent as
Calculating
- Using value of and the number of moles
- Used experimentally
- Using Hess’ Law
- Using Bond energies
- Using Standard heats of formation (at )
- Potential energy diagrams
When is positive, it’s an exothermic reaction. If it’s negative, it’s an endothermic reaction.
If we are calculating q for the surroundings, then we would switch to from
Hess’s Law - Finding Enthalpy and heats of formation
of a reaction can be calculated algebraically using various reactions with known enthalpies
- equations can be reversed; the stays the same but the sign changes
Heats of formations
1/x
min 10 transformations