Unit 1 - Energy
2025-02-04
1
12
… mercury shc 0.14g/J
2025-02-06
375g -15C → gas @ 125C
125—15
2025-02-07
38g sample of ice at -11c is placed into 214g of water at 56c
find final temp
water:
ice:
where is 56C water and is the warmed ice
34.7c
2
5.022g of NaH reacts
5.022 / 84.007 = 0.0597807326
80 * 4.19 * 18.6 - 28.4
= -3284.96
3284.96 / 0.0597807326
55kJ/mol
Why is the
2025-02-10
Unit 2
l
C
1-4
2025-02-28
Reaction Steps
Overall Reaction
Rate Law
Since Step 2 is the rate-determining step:
Expressing in terms of from Step 1:
2025-03-04
I = Catelyist
IO = Intermid
2025-03-24
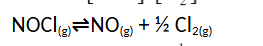
- The equilibrium constant for the equilibrium CO(g) + H2O(g)⇌CO2(g) + H2(g) is 302 at 600k . What is the value of the equilibrium constant for the reverse reaction at the same temperature
3
PCL5 <> PCL3 + CL2
At the equilibrium point in the decomposition of phosphorus pentachloride to chlorine and phosphorus trichloride, the following concentrations are obtained: 0.010 mol/L PCl5, 0.15 mol/L PCl3 and 0.37 mol/L Cl2. Determine the Keq for the reaction
4
The colourless gas dinitrogen tetroxide decomposes to the brown coloured air pollutant nitrogen dioxide and exists in equilibrium. A 0.125 mol sample of dinitrogen tetroxide is introduced into a 1.00 L container and allowed to decompose at a given temperature. When equilibrium is reached, the concentration of the dinitrogen tetroxide is 0.0750 mol/L. What is the value of Keq for this reaction
0.0750 vs
| N2O4 | 2NO2 | |
|---|---|---|
| I | 0.125 | 0 |
| C | 0.05 | 0.1 |
| E | 0.0750 | 0.1 |
11
At 100°C the reaction below has an equilibrium constant, Keq, value of 2.2x10-10. If 1.00 mol of phosgene, COCl2, is placed in a 10.0 L flask, calculate the concentration of carbon monoxide at equilibrium
| COCL2 | CO | CL2 | |
|---|---|---|---|
| I | 0.1 | 0 | 0 |
| C | -x | +x | +x |
| E | 0.1-x | x | x |
the concentration is approx 4.69 x 10⁻⁶ M
2025-03-25
Lead KSP
\begin{align} & \frac{0.85g}{100 mL} \\ & M_{Pbl_{4}} = \frac{714.8g}{mol} \\ & 0.85g \to 0.001189 mol \\ & 100mL = 0.100 L \\ & \therefore \text{ the concentration at equilibrium is 1.2 * 10\^\;-2 mol/L} \end{align}Determine the ion concentrations of a saturated iron (II) hydroxide solution, where solubility is 0.72 g/100 mL. Solve to find the Ksp.
1 Write balanced chemical equations and the for the following dissolving in water
a) Sodium Sulfide
b) Calcium Iodide
c) Lithium Carbonate
d) Iron (II) sulfate
e) cobalt (II) nitrate
f) barium phosphate
5. What are the ion concentrations in saturated solutions of the following
Lead (II) Iodide TODO RECHECK THIS
6 calculate the K sp values of the substances below from their solubility in water
a) thallium(l) chloride, 3.4g/L @ 25c
2025-03-26
solve for x and todo
Sample problem
425mL of 0.15M calcium Chloride
420mL of 0.25M Silver nitrate
Determine:
- a) If a precipitate will form
- b) The concetration of all ions at equilbirium
- c) the mass of the precipitate
- figure out which product is least soluable (would precipitate out first)
- CaNo3 is soluable
- AgCl
-
- To see if a preipiate will form we need to calculate the Q and compare to the Ksp of AgC
- Total volume = 425mL + 450Ml → 0.875 L
- mol Ag+ = C*V = 0.1125 mol
- mol Cl (425mL of 0.15M) * 2 = 0.1275mol
- [ag+] 0.1125 mol / 0.875 L= 0.12857
- [cl-] 0.1275 mol / 0.875 L = 0.145714
b)
- mol ca2+ 0.425L * 0.15M = 0.06375 mol
- = 0.06375 / 0.875 = … todo
c) AgCl is the precipitate
could total - could disolve = precipitated agcl
2025-04-03
two
| NH3 | H2O | NH4 | OH- | |
|---|---|---|---|---|
| initial | 0.38 | - | 0 | 0 |
| change | -x | - | +x | +x |
| eq | 0.38 | x | x |
2025-04-08
Calculate the pH of 0.56M of calcium formate K_a of Formic acid () is
| M | 1 | - | 1 | 1 |
|---|---|---|---|---|
| I | 1.12 | - | 0 | 0 |
| C | -x | - | +x | +x |
| E | 1.12-x | - | x | x |
Calculate the pH of 0.45M solution of NH4NO3 (kb of ammonia is )
0.45-x | x | x
2025-04-09
Find the pH of 253ml of a 4.010^-3 M soltion of HCL ans 12mL of a 3.010^-2 solution of NaOH
2025-04-10
0.150* 0.220 = 0.033 mol
0.440 KOH
150mL 0.220M
0.033 / 0440mol/L = 0.075L = 75mL (why!!>)
| item | C6H5COO- | H2O | OH | C6H5OOH |
|---|---|---|---|---|
| I | 0.147M | - | 0 | 0 |
| C | -x | - | +x | +x |
| E | 0.147-x | - | x | x |
Kb = 110^-14 / 6.310
x^2 =
x = 4.83*10^-6
pOH = -log[OH-] = 5.316
PH = 14 - 5.316 = 8.684
- What’s the pH of a 1.5M NaF solution. Kb of HF is
#todo