Ecology
TERMS
-
ECOLOGY
How organisms interact with each other and their environment
-
ECOSYSTEM
a self-regulating system in which living (biotic) and non-living (abiotic) things interact.
-
SUSTAINABLE (in regards to ecosystem)
The ability for the ecosystem in its current state indefinitely
-
FOOD WEB
Essentially a food chain but it’s more realistic and has multiple paths
E.G. an animal will eat more than one food and more then one thing will eat it.
FACTS
- 10% of food eaten is actually used
Electricity
Static
Everything starts off neutral
How to draw charge diagrams:
- Neutral: same number of plus and minus signs
- Positive: more plus signs
- Negative: more negative signs
PROTRONS NEVER MOVE: don’t change plus signs
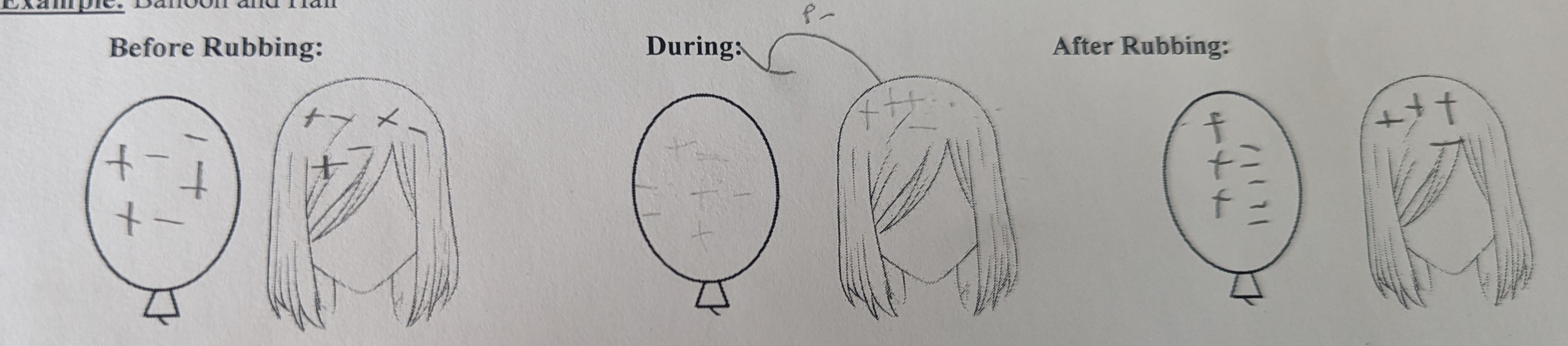
Static electricity: an imbalance of electrons and protons
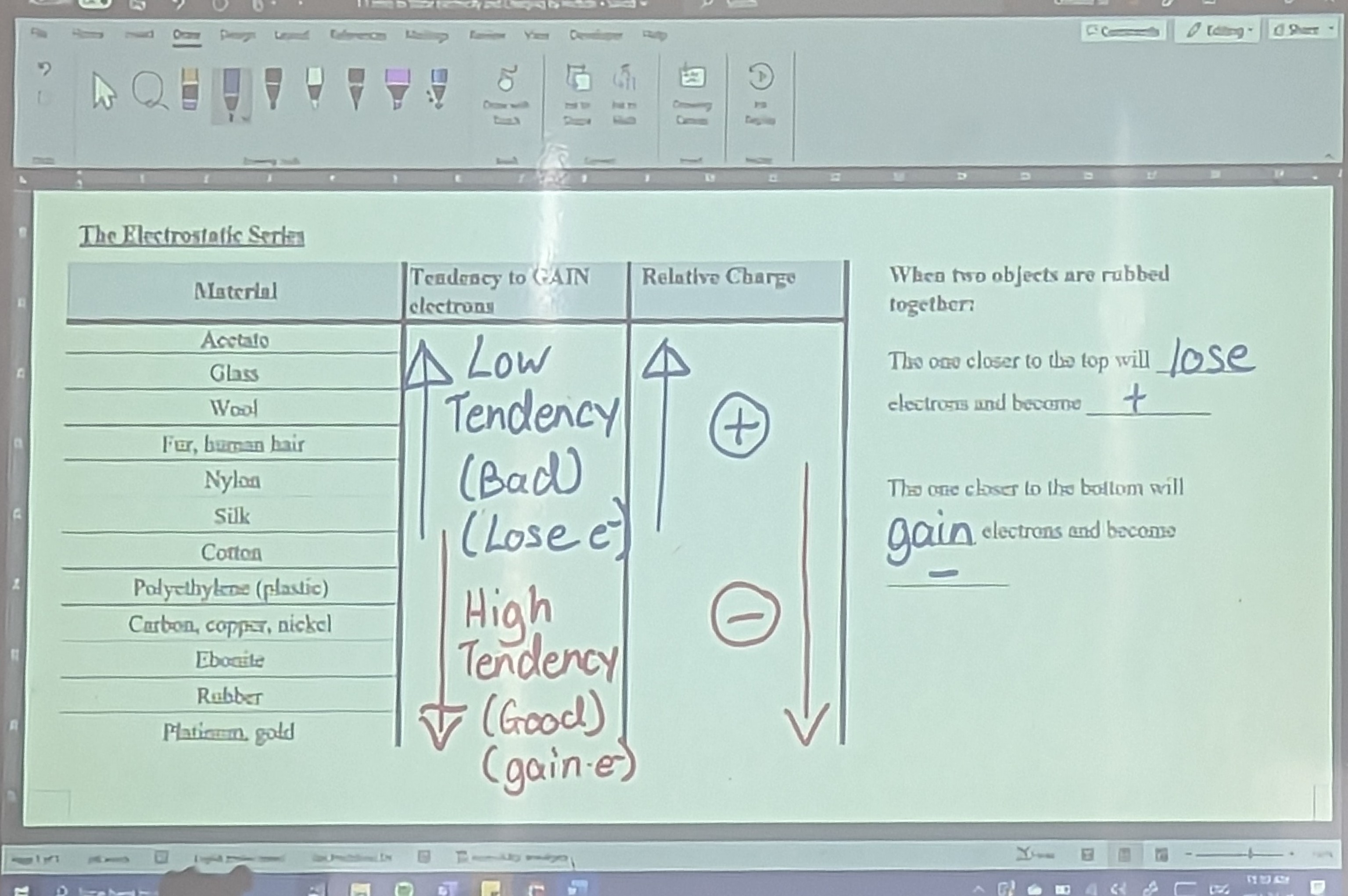
How to know which object gains electrons and which loses electrons: use the electrostatic series which is a list of how strongly common materials attracts electrons
The higher a material on the electrostatic series the worse it is at gaining electrons (lower always gains and higher always loses)
Two identically charged objects repel (+ & + or - & -)
Two differently charged objects attract (+ & - or - & + )
Intro to current electricity
Current electricity
- Electrons carry energy
- A build up or negative static electricity is like a built up or potential energy
- BUT in order to USE the that stored energy, the elections most be moving
- The flow (or movement) of course electric charges is called ELECTRIC CURRENT
- In order for electrons to flow, they need a complete circuit.
The four parts of a circuit
-
Electrical source
A device that energies electrons
I.E. a battery or generator..
-
Electrical load
A device that converts electrical energy into a another useable form
I.e. a lightbulb, tv, electric stove..
-
Control device
A device to control the flow of electricity
“open” means it’s off
“closed” means current can flow (on)
E.g. a switch, light switch, fuse or a breaker
-
Electrical conductors
Wires used to provide a path for current to flow.
I.E. a copper wire
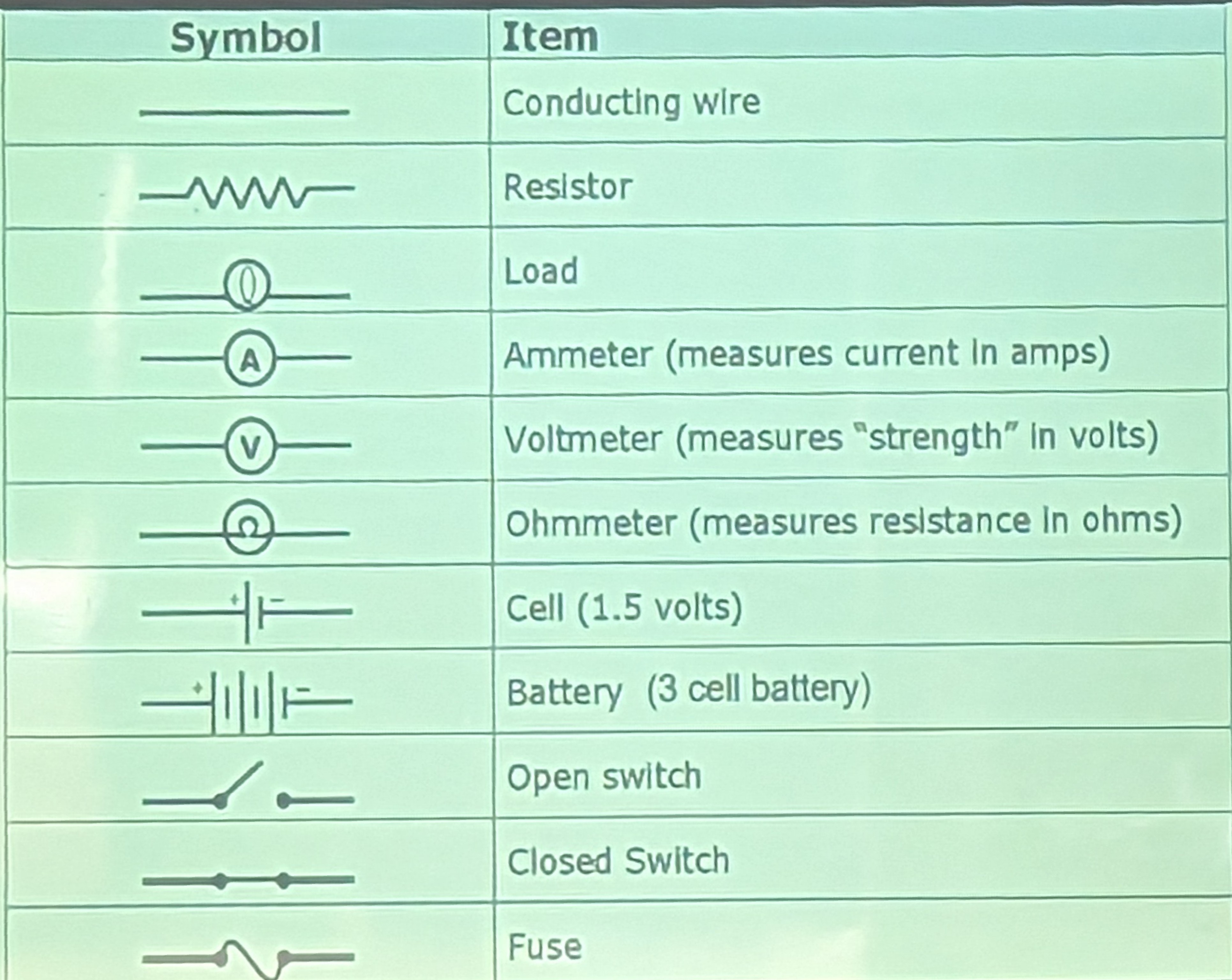
Electrical diagrams
- Batteries - long line is positive, small is negative. And electrons always flow from the negative terminal.
- Resistors, just dampens the flow, doesn’t stop: e.g. a lightbulb would be dimmer
Writing circuits
- Series: there is only one wire and it flows through all of loads
- Parallel: there are multiple different wires/paths in the circuit (i.e. one wire doesn’t pass through every load)
Resistance
- Loads always create more resistance then conductors
- Current is dependent on battery/resistance
Terms
- Voltage - difference in potential energy. Meaning the amount of energy the battery gives the electrons and the amount of energy the electrons give the load)
- Amperage - The amount of current flowing through one point in the circuit (measured in Columbs) per second
Difference in circuits
- In series: voltage / amps splits between loads. (Generally voltage/loads)
- In parallel: voltage splits, so each lightbulb simply gets less electrons flow to it
Meters
- Ammeter
- Place anywhere in a series part of the circuit
- Voltmeter
- Place on either side of a load In parallel
- Why? To measure DIFFERENCE in voltage (energy)
- Place on either side of a load In parallel
Formula’s
- V (voltage in volts)
- I (current in amps) (multiply answer by 1000 to get milliamps)
- R (resistance in ohms)
Internal circuitry (energy transformation and efficiency)
No
- Energy cannot be created or destroyed ONLY transformed
Types of energy
-
Electrical
-
Some electrical energy is always lost due to resistance and converted to heat.
Efficiency formula
-
-
Chemical
E.g. nuclear
-
Kinetic
-
Mechanical
Electrical power
Physics
Non-matter includes the light from a torch, the heat from a fire, and the sound of a police siren.
You cannot hold, taste, or smell these things.
Chem
Matter
Important
Matter is anything that takes up space and can be weighed/have mass
-
Examples of matter
Anything that takes up space is called matter. Air, water, rocks, and even people are examples of matter. Different types of matter can be described by their mass. The mass of an object is the amount of material that makes up the object.
Classifications
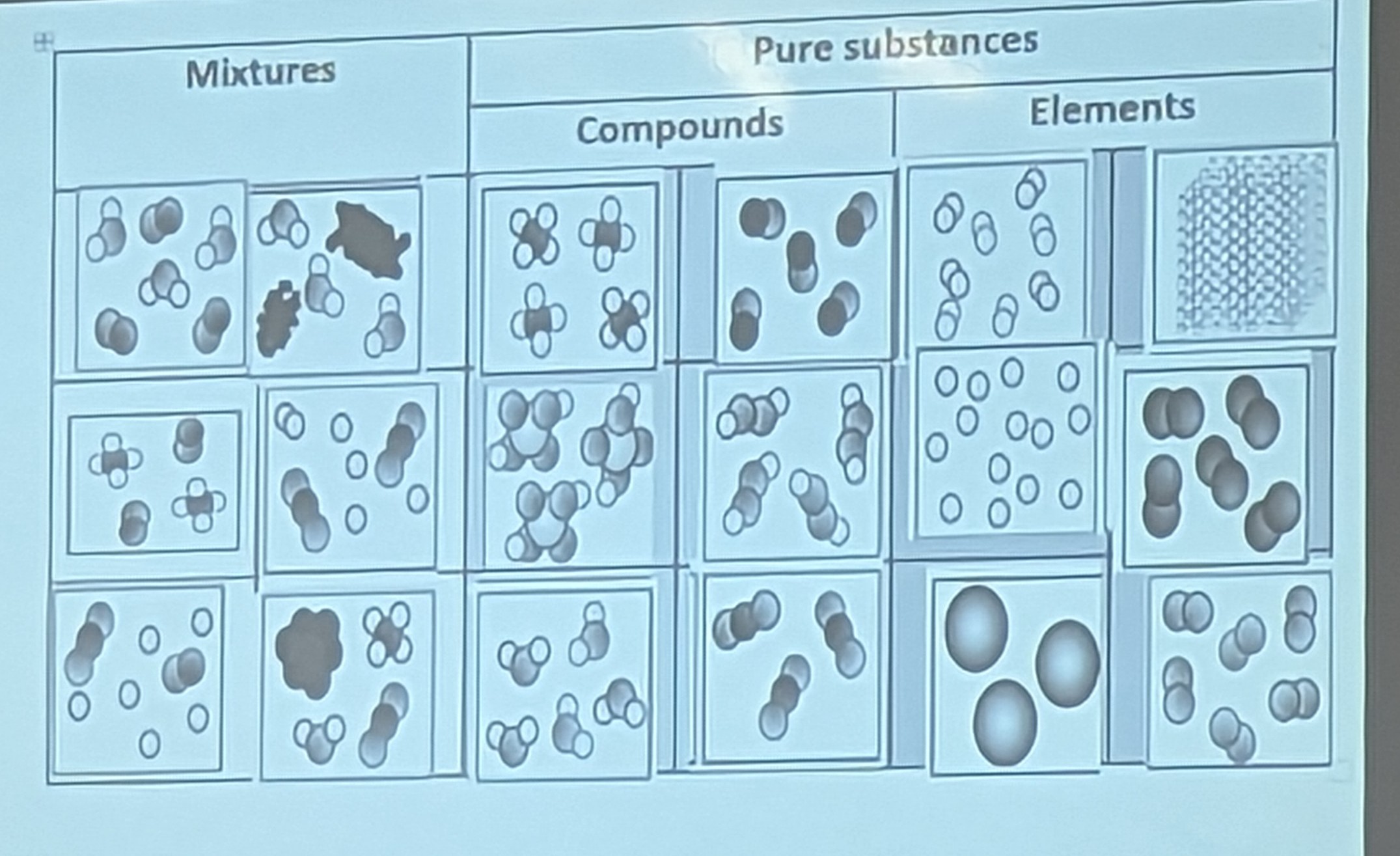
-
Pure substance
A substance made of only one type of particle
Example: water
Types of pure substances: elements and compounds
-
Mixture
A substance made up of at least two different types of particles
Example: milk, cranberry juice
-
Mechanical mixture (heterogeneous)
A mixture in which you can distinguish different types of matter
Under a microscope it looks like groups of elements
Examples: milk, chocolate chip cookies
Any non clear liquid
-
Solution (homogeneous)
A uniform mixture of two more more substances such as a alloy
For liquids if you can’t see through it it’s heterogeneous
Under a microscope it looks well dispersed.
Examples:
- 18k gold (gold + copper)
- Apple juice (Sugar, water and fruit extract)
- Gas mixture (iodine gas + air)
- Air (nitrogen, oxygen, hydrogen, carbon dioxide, …)
-
-
Colloid
a homogeneous noncrystalline substance consisting of large molecules or ultramicroscopic particles of one substance dispersed through a second substance.
Colloids include gels such as jello, and emulsions; the particles do not settle, and cannot be separated out by ordinary filtering or centrifuging like those in a suspension.
-
Physical Properties
Important
A characteristic of matter that is not associated with a change in its chemical composition.
-
Melting point
The point at which a solid turns into a liquid
water freezes at 0 degrees
-
Boiling point
The temperature at which a liquid turns into a gas
Water’s is 100C
Bromine is 58.6C
-
Texture
Describes how the surface of a object feels
-
Lustre
Describes how well a object reflects light
-
Conductivity
The ability of a substance to conduct heat or electricity
E. G. Aluminum is a good conductor of heat and plastic is not.
-
Density
The ratio of the mass of a substance to the volume that it occupies
The greater the mass of a substance per unit volume, the more dense.
-
Solubility
The measure of the ability of a substance to disolve in another substance
-
Malleability
The ability of an object be pounded, rolled or deformed without losing its strength
-
Viscosity
Measure of how easily a fluid flows.
The more viscous a liquid is, the slower it flows.
-
Hardness
Refers to the ability of a substance to be
scratchedHarder substances can scratch sufter substances, but softer
substances cannot scratch harder substances Diamond scratched glass, therefore diamond is harder than glass. -
Qualitative vs quantitative
Qualitative Properties
A property of a substance that
is not measured and does not
have a numerical valueExamples: colour, odour, and texture
Quantitative Properties
A property of a substance that is measured and has numerical valueExamples: temperature, height, and mass
-
COMMON UNITS
Volume: litre (L) milliliters (ml)
Weight: kilogram (kg) grams (g)
Chemical properties
Important
A chemical property describes the ability of a substance to react with another substance and form one or more new substances with new properties.
-
Combustibility
Describes the ability of a substance to burn in air producing heat and light
-
Decomposition
Describes the change that can occur when a substance is broken down into the parts that make it up
When an electrical current passes through water, it causes it to break down into oxygen and hydrogen gas -
Reactivity with Oxygen
Describes the change that can occur when a substance is exposed to oxygen
The flesh of somekinds of fruit turns brown when exposed to oxygen in the air -
Reactivity with Acids
Describes the change that can occur when a substance is exposed to acid
Many metals, such as zinc, will react with acid to form hydrogen gas -
Reactivity with other substances
Describes the change that can occur when one substance reacts with another substance
When some solutions are mixed together, they form a solid called a precipitate, which is a new substance. -
Is it a physical or
A chemical change involves the formation of something new. These clues tell you that a new substance has formed
-
The substance changes colour
Bubbles form, telling you a new gas has been produced -
A new odour forms, telling you a new gas has been formed
-
A new solid (a precipitate forms)
-
Energy in the form of heat, light and/or sound is released when the substances are mixed
-
Changes in matter
Important
Matter can change in two different ways: Chemical or Physical
-
Physical changes
Alters only the form or state of a substance. No new substance is created.
Examples:
- Dissolving Suger in water
- Breaking glass
- Chopping wood
- Sublimation of dry ice
-
Chemical changes
Changes a substance into a new substance that has different physical and chemical properties
Examples:
-
Baking a cake
-
Burning wood
-
Souring milk
-
Photosynthesis
-
Rusting
-
-
How to tell if the substance has changed forms
CHANGES IN properties
- Changes in color (e.g. copper)
- Formation of bubbles
- Changes in odor
- Formation of a precipitate
CHANGES IN ENERGY
- Warming or cooking
- The release of light
-
How to test for the type of gas in bubbles
- Test for Oxygen Gas (O2) –
Glowing splint → reignite
- Test for Hydrogen Gas (H2) –
Flaming split → “pop”
-
Test for Carbon Dioxide Gas (CO2) – Flaming splint → go out
Particle theory
-
All matter is made up of extremely small particles.
-
All particles of one substance
are the same. -
There are spaces between
the particles. -
The particles are always
moving. -
The more energy that particles have, the faster they move.
-
There are attractive forces among particles. These forces are stronger when the particles are closer together.
-
Water is 1g/cm³
ATOMS
Terms
-
Valence
outer shell/number of electrons it can have in the outermost ring
The octet rule
-
Element that have a full shell and want to be insert
- atoms want to be full
-
Atoms of elements other than the noble gases do not have full valence electrons (g#18)
-
Atoms that do not have full valence shells tend to combine a way that they achieve full valence shells
Ways to combine:
-
Atoms can gain electrons
-
Atoms can lose electrons
-
Atoms can share electrons
-
Formation of Ions:
-
Ions: a charged entity formed when atoms gain or lose electrons
-
Atoms can form either positive or negative ions
-
Metals likely to lose electrons and form positive ions are called cation (positive charge)
-
Non-metals are likely to gain electrons and form negative ions called anion (negative charge)
Electrons are negative
Protons are Positive
-
Ionic compounds> [!important]
Always has to contain a metal
-
Ionic compounds = transfer (give and take) of electrons
-
lonic compounds are usually formed when metals bond with non-metals
-
They are usually Crystalline solids (e.g. table salt)
-
They have high melting points and high boiling points.
-
They are usually soluble in water but insoluble in organic solvents.
-
They conduct electricity when dissolved in water or when melted.
Involves
- Cations (+)
- Anions (-)
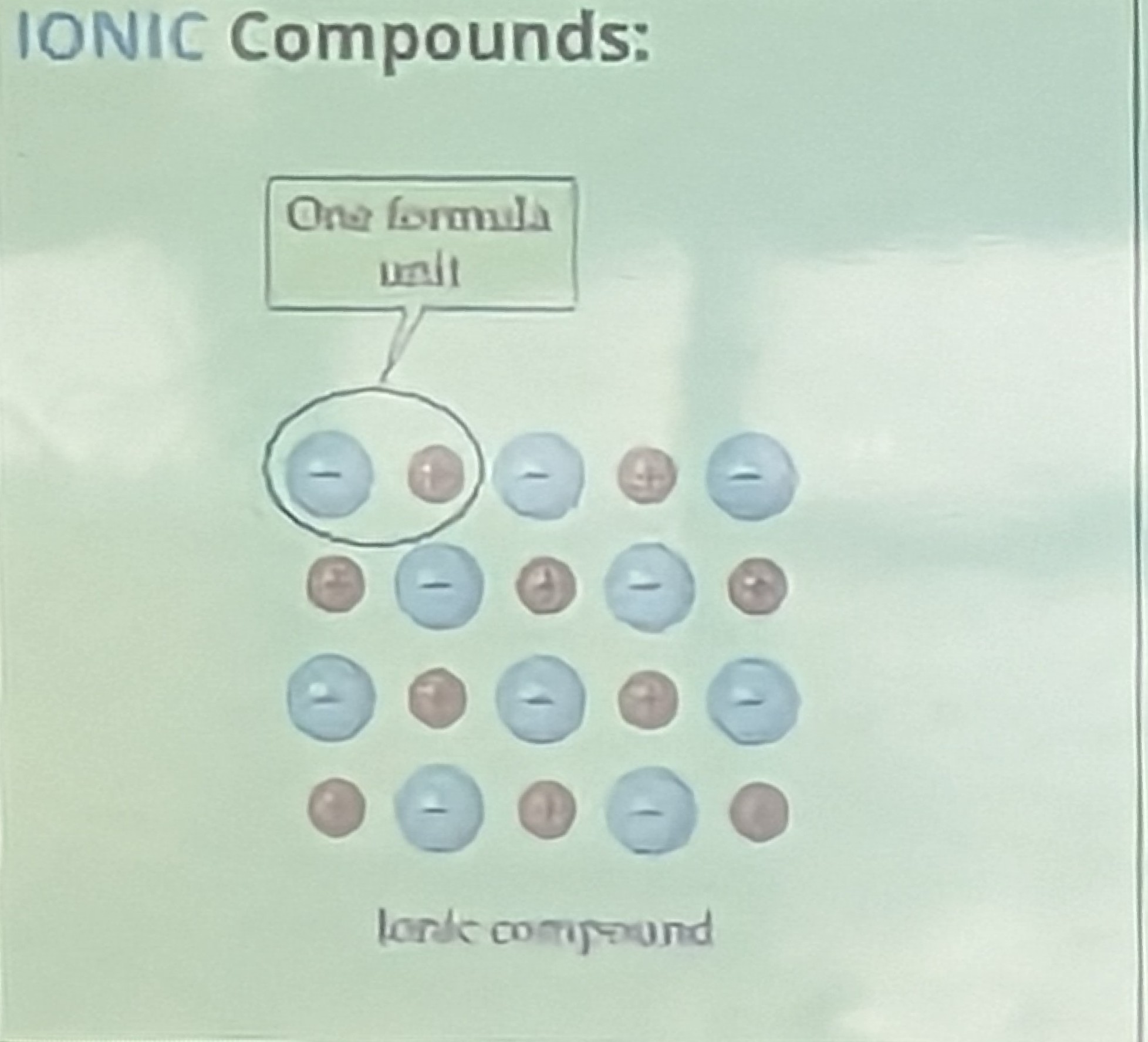
-
-
Molecular/Covalent Compounds
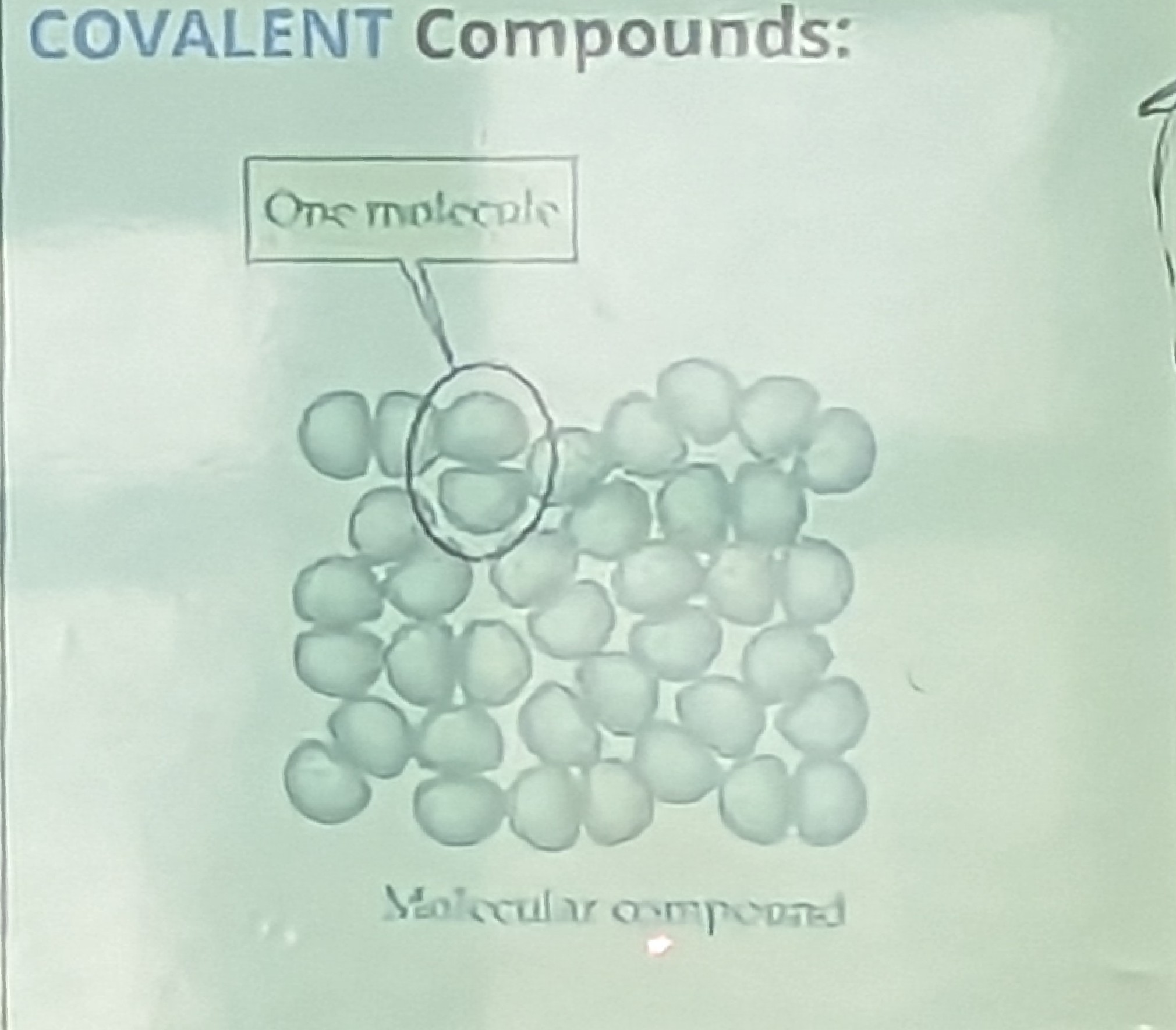
- Molecular compounds are made of covalent bonds
- Covalent bonds = sharing of electrons
- Covalent compounds are usually formed when non- metals band together
involves:
non-metals
When you gain smth you go negative and positive is lose.
-
Inonic
-
Diagrams
-
Number of protons is equal to the atomic number
-
The same applies with electrons if it’s a stable
-
Bohr
-
Lewis
-
-
Atomic bonds
How to tell if a compounds Ionic or covekent